Acids bases & ph worksheet – Acids, Bases, and pH Worksheet: Embark on a captivating journey into the fascinating realm of chemistry, where acids and bases dance in a delicate balance, shaping our world in countless ways. This comprehensive worksheet unravels the secrets of these fundamental concepts, empowering you with a deep understanding of their properties, behaviors, and applications.
Delve into the intricacies of pH, the scale that measures the acidity or basicity of a substance, and discover how it plays a crucial role in various scientific fields and everyday life. Explore the diverse types of acids and bases, from strong to weak, monoprotic to polyprotic, and unravel the fascinating reactions that occur when they interact.
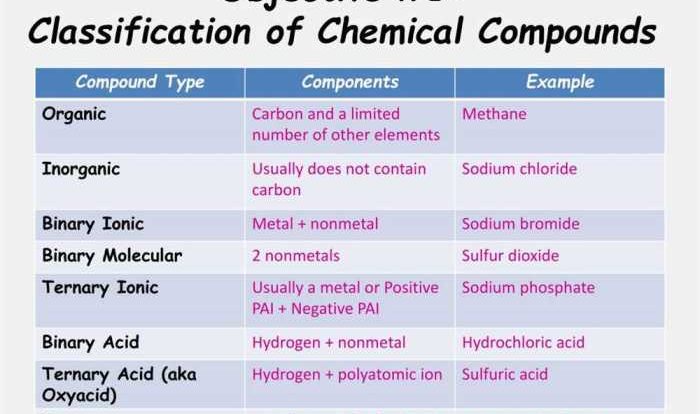
Acids and Bases: Acids Bases & Ph Worksheet
Acids and bases are two important classes of chemical compounds that play a crucial role in various chemical reactions and processes. They are defined based on their properties and characteristics.
Acids are substances that, when dissolved in water, release hydrogen ions (H+) and have a pH value less than 7. They are typically sour in taste and can react with metals to produce hydrogen gas. Common examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3).
Bases, on the other hand, are substances that, when dissolved in water, release hydroxide ions (OH-) and have a pH value greater than 7. They are usually bitter in taste and can react with acids to form salts. Examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).
Types of Acids and Bases
Acids and bases can be further classified into different types based on their strength and the number of hydrogen or hydroxide ions they can release.
- Strong acidsare acids that completely dissociate in water, releasing all of their hydrogen ions. Examples include hydrochloric acid (HCl) and sulfuric acid (H2SO4).
- Weak acidsare acids that only partially dissociate in water, releasing only a small fraction of their hydrogen ions. Examples include acetic acid (CH3COOH) and carbonic acid (H2CO3).
- Monoprotic acidsare acids that can release only one hydrogen ion per molecule. Examples include hydrochloric acid (HCl) and nitric acid (HNO3).
- Polyprotic acidsare acids that can release more than one hydrogen ion per molecule. Examples include sulfuric acid (H2SO4) and phosphoric acid (H3PO4).
- Strong basesare bases that completely dissociate in water, releasing all of their hydroxide ions. Examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
- Weak basesare bases that only partially dissociate in water, releasing only a small fraction of their hydroxide ions. Examples include ammonia (NH3) and calcium hydroxide (Ca(OH)2).
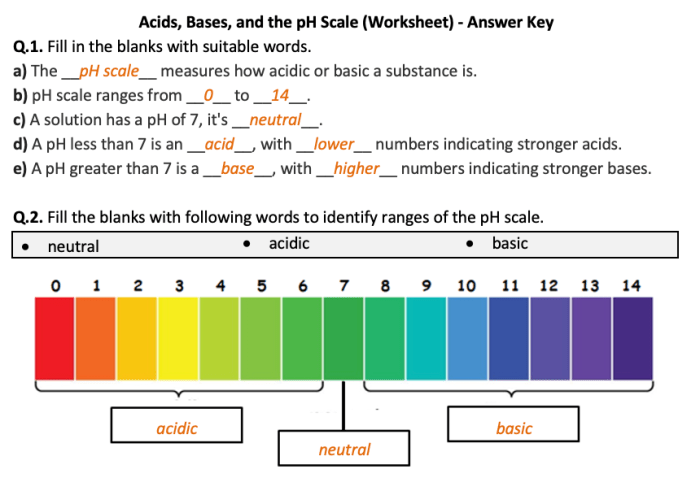
pH and the pH Scale
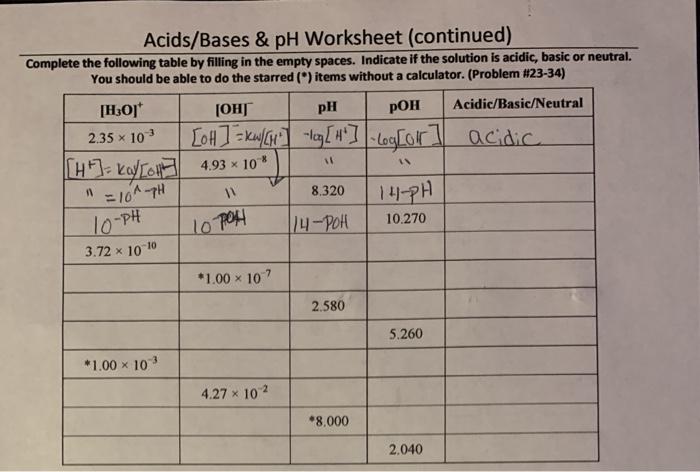
pH is a measure of the acidity or basicity of a solution. It is defined as the negative logarithm of the hydrogen ion concentration in moles per liter.
The pH scale ranges from 0 to 14, with 7 being neutral. Values below 7 indicate acidity, while values above 7 indicate basicity.
pH Values of Common Substances
- Battery acid: 0
- Stomach acid: 1-2
- Lemon juice: 2-3
- Orange juice: 3-4
- Pure water: 7
- Seawater: 8
- Household ammonia: 11-12
- Bleach: 12-13
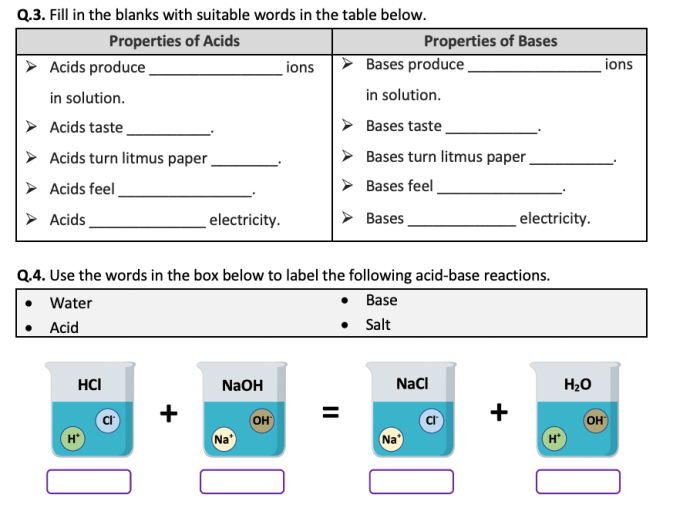
Acid-Base Reactions
Acid-base reactions are chemical reactions that involve the transfer of protons (H+) between an acid and a base. Acids are substances that donate protons, while bases are substances that accept protons.
The most common type of acid-base reaction is a neutralization reaction. In a neutralization reaction, an acid and a base react to form a salt and water. For example, the reaction of hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H2O):
HCl + NaOH → NaCl + H2O
Acid-base reactions can also be used to prepare other compounds. For example, the reaction of sulfuric acid (H2SO4) and copper oxide (CuO) produces copper sulfate (CuSO4) and water:
H2SO4 + CuO → CuSO4 + H2O
Acid-base reactions are important in many chemical processes, including the production of fertilizers, pharmaceuticals, and plastics.
Titrations
Titrations are a fundamental technique used in analytical chemistry to determine the concentration of an unknown solution by reacting it with a solution of known concentration. Acid-base titrations are a specific type of titration used to determine the concentration of an acid or base.
The principle of an acid-base titration is to add a known volume of a base to an unknown volume of acid (or vice versa) until the reaction is complete. The point at which the reaction is complete is called the equivalence point.
At the equivalence point, the moles of acid and base are equal, and the solution is neutral (pH = 7).
Steps Involved in Performing an Acid-Base Titration
- Prepare a solution of known concentration, called the titrant.
- Measure an accurately known volume of the unknown solution into a flask.
- Add a few drops of an indicator to the unknown solution.
- Slowly add the titrant to the unknown solution, while swirling the flask constantly.
- Observe the color change of the indicator to determine the equivalence point.
- Record the volume of titrant used to reach the equivalence point.
Acid-Base Titration Calculations
The concentration of the unknown solution can be calculated using the following formula:“`M1V1 = M2V2“`where:* M1 is the concentration of the titrant
- V1 is the volume of titrant used
- M2 is the concentration of the unknown solution
- V2 is the volume of the unknown solution
For example, if 25.0 mL of a 0.100 M NaOH solution is used to neutralize 50.0 mL of an unknown HCl solution, the concentration of the HCl solution can be calculated as follows:“`
Acids, bases, and pH can be confusing topics, but they don’t have to be. If you’re looking for a fun way to learn about them, check out this worksheet. It’s full of interactive exercises and quizzes that will help you understand these concepts in no time.
And if you need a break from science, you can always take a break to ponder the age-old question: Mike Brady or Phil Dunphy? Click here to vote for your favorite TV dad. Then come back and finish learning about acids, bases, and pH!
- 100 M
- 25.0 mL = M2
- 50.0 mL
M2 = 0.0500 M“`Therefore, the concentration of the unknown HCl solution is 0.0500 M.
Applications of Acids and Bases
Acids and bases play a vital role in various aspects of everyday life and industries. They are used in numerous products and processes, from maintaining the health of living organisms to manufacturing essential materials. Understanding their applications helps us appreciate the significance of acids and bases in our world.
Batteries
Acids and bases are crucial components in batteries, devices that convert chemical energy into electrical energy. Lead-acid batteries, commonly found in vehicles, use sulfuric acid as an electrolyte, facilitating the chemical reactions that generate electricity. Similarly, lithium-ion batteries, used in electronic devices, rely on electrolytes containing acids or bases to enable ion movement and power generation.
Fertilizers, Acids bases & ph worksheet
Acids are essential for the production of fertilizers, which provide nutrients to plants. Nitric acid and sulfuric acid are used to synthesize nitrogen-based fertilizers, such as ammonium nitrate and urea, which are vital for crop growth and agricultural productivity. These acids react with ammonia to form salts that serve as nitrogen sources for plants.
Household Cleaners
Acids and bases are widely used in household cleaning products. Acids, such as hydrochloric acid and acetic acid (vinegar), are effective in removing mineral deposits, stains, and dirt. Bases, like sodium hydroxide and ammonia, are commonly used as degreasers and disinfectants.
Their ability to neutralize acids and dissolve organic matter makes them effective cleaning agents.
pH Control
Maintaining optimal pH levels is crucial in various applications. In living organisms, pH plays a vital role in cellular processes, enzyme activity, and overall health. For instance, the pH of blood is tightly regulated within a narrow range to ensure proper functioning of enzymes and physiological processes.
Similarly, in industries, pH control is essential in processes like water treatment, chemical synthesis, and food preservation. Adjusting pH levels helps optimize reactions, prevent corrosion, and ensure product quality.
Popular Questions
What is the difference between an acid and a base?
Acids release hydrogen ions (H+) in water, while bases release hydroxide ions (OH-) in water.
What is the pH scale?
The pH scale measures the acidity or basicity of a substance, ranging from 0 to 14, with 7 being neutral, values below 7 indicating acidity, and values above 7 indicating basicity.
What is a neutralization reaction?
A neutralization reaction occurs when an acid and a base react to form a salt and water, releasing heat in the process.
What are the applications of acids and bases?
Acids and bases have numerous applications in everyday life and industries, including in batteries, fertilizers, household cleaners, and the production of various chemicals.