Welcome to Chapter 12.1 Stoichiometry Answer Key, your ultimate resource for understanding the fundamental principles of stoichiometry. This comprehensive guide provides clear and concise explanations, engaging examples, and practical applications to help you master this essential chemistry concept.
Throughout this guide, we will explore the basics of stoichiometry, including mole-to-mole conversions, mass-to-mass conversions, solution stoichiometry, titration stoichiometry, and gas stoichiometry. Our goal is to equip you with the knowledge and skills necessary to confidently solve stoichiometry problems and apply them to real-world scenarios.
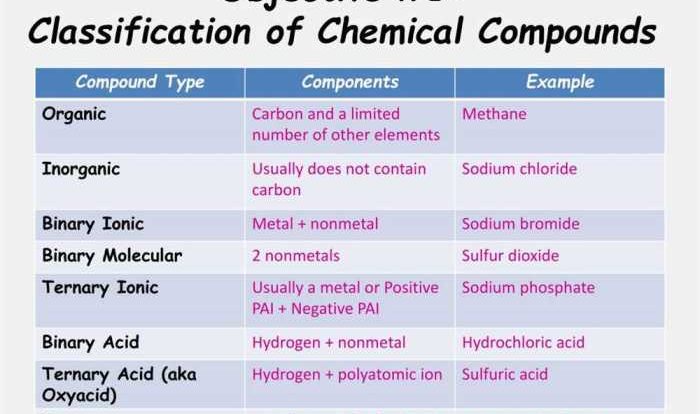
Stoichiometry Concepts
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It provides a framework for predicting the amounts of reactants and products involved in a given reaction, enabling scientists to make accurate predictions and calculations.
Chemical equations represent stoichiometric relationships symbolically. For example, the combustion of methane can be represented as:
CH4+ 2O 2→ CO 2+ 2H 2O
This equation indicates that one molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water. The coefficients in front of each chemical formula represent the stoichiometric ratios, which are the relative amounts of reactants and products involved in the reaction.
Molar mass, expressed in grams per mole (g/mol), is a crucial concept in stoichiometry. It represents the mass of one mole of a substance and is calculated by adding the atomic masses of all atoms in the molecular formula.
Mole-to-Mole Conversions
Stoichiometry enables the conversion between moles and grams of a substance. The mole-to-gram conversion factor is the molar mass of the substance. For example, to convert 2 moles of methane to grams, we multiply by its molar mass (16 g/mol):
moles CH4× 16 g/mol = 32 g CH 4
In chemical reactions, the limiting reactant is the reactant that is completely consumed, while the excess reactant is the reactant that remains after the reaction is complete. The stoichiometric ratios determine which reactant is limiting and which is in excess.
For example, consider the reaction between 2 moles of methane and 4 moles of oxygen:
CH4+ 2O 2→ CO 2+ 2H 2O
Using the mole-to-mole ratios, we find that 1 mole of methane reacts with 2 moles of oxygen. Since we have 2 moles of methane and 4 moles of oxygen, methane is the limiting reactant and oxygen is in excess.
Mass-to-Mass Conversions
Stoichiometry also allows for conversions between masses of reactants and products. The mass-to-mass conversion factor is the molar mass of the substance multiplied by the stoichiometric ratio.
For example, to convert 10 g of methane to grams of carbon dioxide, we first convert 10 g to moles using the molar mass of methane (16 g/mol):
g CH4× (1 mol CH 4/ 16 g CH 4) = 0.625 moles CH 4
Then, we use the stoichiometric ratio from the balanced equation (1 mole CH 4produces 1 mole CO 2) and multiply by the molar mass of carbon dioxide (44 g/mol):
625 moles CH4× (1 mole CO 2/ 1 mole CH 4) × 44 g/mol CO 2= 27.5 g CO 2
Solution Stoichiometry
Solution stoichiometry deals with the quantitative relationships in solutions. Molarity (M) is a measure of the concentration of a solution, defined as the number of moles of solute per liter of solution.
To calculate the concentration of a solution, we divide the number of moles of solute by the volume of the solution in liters. For example, a solution containing 0.1 moles of sodium chloride (NaCl) in 1 liter of solution has a concentration of 0.1 M.
Dilution is a process of decreasing the concentration of a solution by adding more solvent. When a solution is diluted, the number of moles of solute remains the same, but the volume of the solution increases.
Titration Stoichiometry, Chapter 12.1 stoichiometry answer key
Titration is a technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration. The equivalence point is reached when the moles of titrant added are stoichiometrically equivalent to the moles of analyte in the unknown solution.
Titration calculations involve using the stoichiometric ratio between the titrant and analyte to determine the unknown concentration. For example, if 25.0 mL of a 0.1 M NaOH solution is required to neutralize 10.0 mL of an unknown HCl solution, the concentration of the unknown HCl solution can be calculated.
Gas Stoichiometry
Gas stoichiometry involves the study of quantitative relationships in reactions involving gases. The ideal gas law, PV = nRT, relates the pressure (P), volume (V), temperature (T), and number of moles (n) of a gas.
Stoichiometry can be used to solve problems involving gas reactions. For example, to determine the volume of oxygen required to react completely with 10.0 g of methane, we can use the ideal gas law and the stoichiometric ratio from the balanced equation.
General Inquiries: Chapter 12.1 Stoichiometry Answer Key
What is stoichiometry?
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions.
How do I convert between moles and grams?
To convert between moles and grams, use the molar mass of the substance. The molar mass is the mass of one mole of a substance, expressed in grams per mole.
What is the limiting reactant in a chemical reaction?
The limiting reactant is the reactant that is completely consumed in a chemical reaction, limiting the amount of product that can be formed.