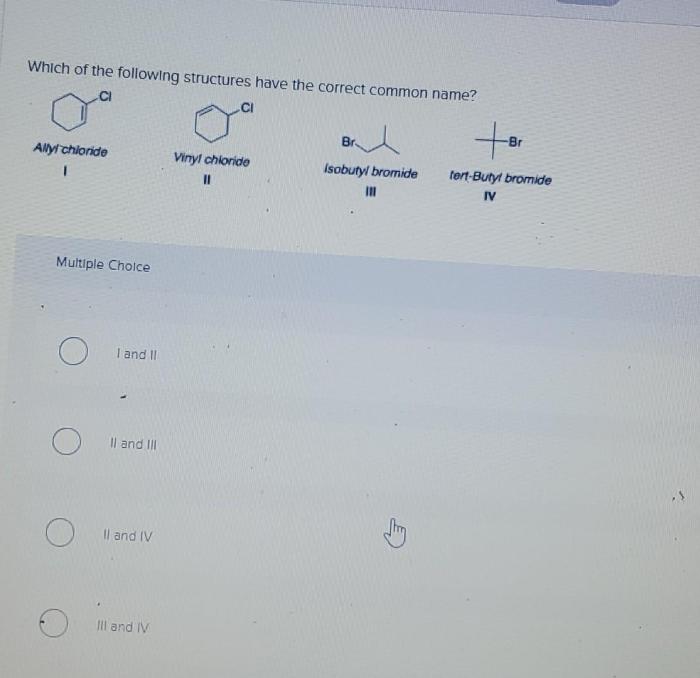
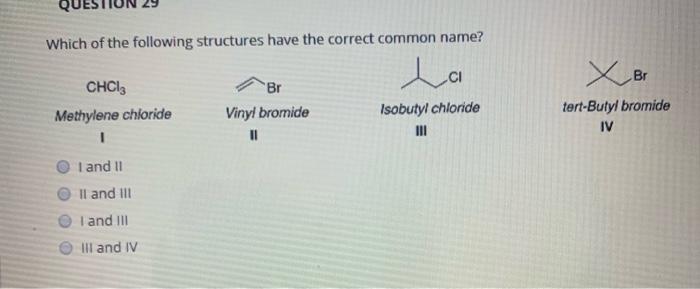
Which of the following structures have the correct common name? This question delves into the intricacies of chemical nomenclature, a fascinating realm where structures and names intertwine. Understanding the principles behind common names empowers chemists to communicate effectively and navigate the vast landscape of chemical compounds.
This guide provides a comprehensive overview of common name structures, exploring the IUPAC guidelines that govern their assignment. We will embark on a journey of structural analysis, identifying functional groups and other key features that shape a molecule’s identity. By comparing structures, we will uncover similarities, differences, and the concept of isomerism.
Common Name Structures
Common names are simplified, non-systematic names for chemical structures. They are often based on the structure or properties of the compound, and can be useful for quick identification and communication. However, common names can be ambiguous and vary depending on the context, so it is important to use IUPAC guidelines when assigning common names.
Some examples of common names and their corresponding structures include:
- Water (H 2O)
- Table salt (NaCl)
- Baking soda (NaHCO 3)
- Vinegar (CH 3COOH)
- Sugar (C 12H 22O 11)
IUPAC guidelines for assigning common names include:
- The name should be as simple and unambiguous as possible.
- The name should reflect the structure of the compound.
- The name should be consistent with the names of other similar compounds.
Structural Analysis
Structural analysis is the process of identifying the functional groups and other key features of a chemical structure. This information can be used to determine the molecular formula and molecular weight of the structure, as well as to predict its properties and reactivity.
To perform structural analysis, it is first necessary to identify the functional groups present in the structure. Functional groups are groups of atoms that have characteristic chemical properties. Some common functional groups include:
- Alkanes (C nH 2n+2)
- Alkenes (C nH 2n)
- Alkynes (C nH 2n-2)
- Alcohols (R-OH)
- Aldehydes (R-CHO)
- Ketones (R-CO-R’)
- Carboxylic acids (R-COOH)
- Amines (R-NH 2)
- Amides (R-CONH 2)
Once the functional groups have been identified, it is possible to determine the molecular formula of the structure. The molecular formula gives the number of atoms of each element present in the structure. To determine the molecular formula, it is necessary to add up the number of atoms of each element in the functional groups.
The molecular weight of a structure is the sum of the atomic weights of the atoms present in the structure. To determine the molecular weight, it is necessary to multiply the number of atoms of each element by the atomic weight of that element and then add up the results.
Comparison of Structures
Comparing two or more chemical structures can be useful for identifying similarities and differences in their functional groups, molecular formulas, and other features. This information can be used to predict the properties and reactivity of the structures, as well as to determine if they are isomers or not.
To compare two or more chemical structures, it is first necessary to identify the functional groups present in each structure. Once the functional groups have been identified, it is possible to compare the molecular formulas and other features of the structures.
If two structures have the same molecular formula but different functional groups, they are said to be isomers. Isomers have the same molecular formula but different structures and properties. There are two main types of isomers: structural isomers and stereoisomers.
- Structural isomers have the same molecular formula but different connectivity of atoms.
- Stereoisomers have the same molecular formula and connectivity of atoms but different spatial arrangements of atoms.
HTML Table Creation
HTML tables are a useful way to display data in a structured format. They can be used to display chemical structures, as well as other types of data.
To create an HTML table, it is necessary to use the following tags:
-
: This tag creates the table.
: This tag creates a row in the table. : This tag creates a cell in the table. For example, the following code creates a table with two rows and three columns:
“`html
Cell 1 Cell 2 Cell 3 Cell 4 Cell 5 Cell 6 “`
The resulting table will look like this:
“`+—+—+—+| Cell 1 | Cell 2 | Cell 3 |+—+—+—+| Cell 4 | Cell 5 | Cell 6 |+—+—+—+“`
Bullet Point Organization
Bullet points are a useful way to organize information in a clear and concise manner. They can be used to list the key points of a discussion, or to provide a summary of a topic.
To create a bullet point list, it is necessary to use the following tags:
-
- : This tag creates an unordered list.
-
- : This tag creates an ordered list.
- : This tag creates a list item.
For example, the following code creates a bullet point list of the key points of a discussion:
“`html
- Point 1
- Point 2
- Point 3
“`
The resulting list will look like this:
“`
- Point 1
- Point 2
- Point 3
“`
Questions Often Asked: Which Of The Following Structures Have The Correct Common Name
What is the purpose of common names for chemical structures?
Common names provide a simplified and recognizable way to refer to chemical compounds, making them easier to remember and use in everyday communication.
How are common names assigned according to IUPAC guidelines?
IUPAC guidelines prioritize simplicity, conciseness, and the use of prefixes to indicate the number of carbon atoms in the parent chain.
What is the difference between a structural formula and a common name?
A structural formula depicts the exact arrangement of atoms in a molecule, while a common name is a simplified and often abbreviated representation of the structure.