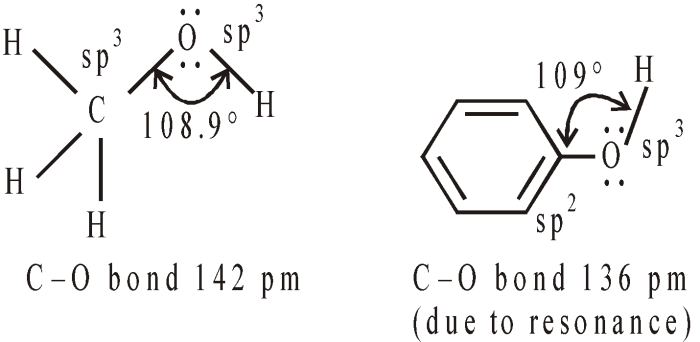
Alcohols ethers and phenols contain oxygen with only single bonds – Alcohols, ethers, and phenols, a trio of organic compounds, share a defining characteristic: they all contain oxygen atoms bonded to carbon atoms by single bonds. This structural feature imparts unique physical and chemical properties to these compounds, distinguishing them from hydrocarbons and other organic molecules.
Their presence in various industrial and commercial applications, coupled with their importance in everyday life, underscores the significance of understanding the chemistry of alcohols, ethers, and phenols.
Structural Characteristics

Alcohols, ethers, and phenols are organic compounds that contain oxygen with only single bonds. This structural feature distinguishes them from other oxygen-containing functional groups, such as aldehydes, ketones, and carboxylic acids, which contain oxygen double bonds.
The presence of oxygen with only single bonds in alcohols, ethers, and phenols results in a specific set of physical and chemical properties.
Examples of molecular structures for each class of compounds:
- Alcohol: CH 3-CH 2-OH (ethanol)
- Ether: CH 3-O-CH 3(dimethyl ether)
- Phenol: C 6H 5-OH (phenol)
Physical Properties
The oxygen-hydrogen bond in alcohols, ethers, and phenols has a significant impact on their physical properties.
- Boiling points:The boiling points of alcohols, ethers, and phenols are generally higher than those of similar hydrocarbons due to the presence of the polar oxygen-hydrogen bond. This bond results in intermolecular hydrogen bonding, which increases the intermolecular forces between molecules and requires more energy to overcome during boiling.
- Solubility:Alcohols and phenols are more soluble in water than ethers because they can form hydrogen bonds with water molecules. Ethers, on the other hand, are less soluble in water due to their nonpolar nature.
- Polarity:The oxygen-hydrogen bond in alcohols and phenols makes these compounds polar. Ethers, however, are nonpolar due to the cancellation of the polar oxygen-carbon bond by the polar carbon-hydrogen bonds.
Chemical Reactivity
Alcohols, ethers, and phenols can undergo a variety of chemical reactions, including:
Nucleophilic Substitution
Alcohols and phenols can undergo nucleophilic substitution reactions, in which a nucleophile (a species with a lone pair of electrons) attacks the electrophilic carbon atom of the alcohol or phenol. This reaction results in the formation of a new bond between the nucleophile and the carbon atom and the breaking of the bond between the carbon atom and the leaving group.
Examples:
- Reaction of an alcohol with a hydroxide ion to form an alkoxide ion:
- Reaction of a phenol with a sodium hydroxide solution to form a phenoxide ion:
CH 3-CH 2-OH + OH –→ CH 3-CH 2-O –+ H 2O
C 6H 5-OH + NaOH → C 6H 5-O –Na ++ H 2O
Electrophilic Addition
Ethers can undergo electrophilic addition reactions, in which an electrophile (a species with a positive charge or a deficiency of electrons) attacks the nucleophilic oxygen atom of the ether. This reaction results in the formation of a new bond between the electrophile and the oxygen atom and the breaking of the bond between the oxygen atom and one of the carbon atoms.
Examples:
- Reaction of an ether with hydrogen bromide to form an alkyl bromide and an alcohol:
- Reaction of an ether with a Lewis acid, such as aluminum chloride, to form an oxonium ion:
CH 3-O-CH 3+ HBr → CH 3-Br + CH 3-OH
CH 3-O-CH 3+ AlCl 3→ [CH 3-O-CH 3-AlCl 3] +
Oxidation, Alcohols ethers and phenols contain oxygen with only single bonds
Alcohols, ethers, and phenols can undergo oxidation reactions, in which they lose electrons to an oxidizing agent. The products of oxidation reactions vary depending on the starting material and the oxidizing agent used.
Examples:
- Oxidation of an alcohol to form an aldehyde or a ketone:
- Oxidation of an ether to form an aldehyde or a ketone:
- Oxidation of a phenol to form a quinone:
CH 3-CH 2-OH + [O] → CH 3-CHO + H 2O
CH 3-O-CH 3+ [O] → CH 3-CHO + CH 3-OH
C 6H 5-OH + [O] → C 6H 4O 2+ H 2O
Applications
Alcohols, ethers, and phenols have a wide range of industrial and commercial applications.
- Alcohols:Alcohols are used as solvents, fuels, and starting materials for other chemical syntheses. Ethanol, for example, is used as a solvent in the production of perfumes, cosmetics, and pharmaceuticals. Methanol is used as a fuel in racing cars and as a starting material for the production of formaldehyde.
- Ethers:Ethers are used as solvents, anesthetics, and starting materials for other chemical syntheses. Diethyl ether, for example, is used as an anesthetic in surgery. Tetrahydrofuran is used as a solvent in the production of pharmaceuticals and other chemicals.
- Phenols:Phenols are used as disinfectants, antiseptics, and starting materials for other chemical syntheses. Phenol, for example, is used as a disinfectant in hospitals and as a starting material for the production of aspirin. Bisphenol A is used as a starting material for the production of polycarbonate plastics.
FAQ Insights: Alcohols Ethers And Phenols Contain Oxygen With Only Single Bonds
What is the key structural feature of alcohols, ethers, and phenols?
The presence of oxygen atoms bonded to carbon atoms by single bonds.
How do the physical properties of alcohols, ethers, and phenols differ from hydrocarbons?
The oxygen-hydrogen bond in these compounds influences their boiling points, solubility, and polarity, making them more polar and less volatile than hydrocarbons.
What are some common chemical reactions involving alcohols, ethers, and phenols?
These compounds can undergo nucleophilic substitution, electrophilic addition, and oxidation reactions, among others.