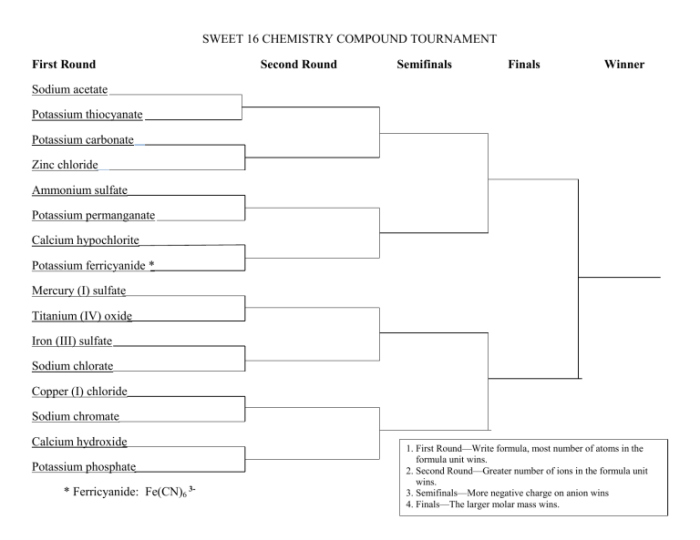
Embarking on the Sweet 16 Chemistry Compound Tournament, we delve into a captivating realm of chemical wonders. This tournament showcases sixteen remarkable compounds, each possessing unique properties and applications that have shaped our world. Join us as we explore their fascinating characteristics, unravel their interactions, and uncover their significance in various fields.
From the fundamental principles of chemical compounds to the intricate details of their reactions and environmental impact, this tournament promises a comprehensive journey through the fascinating world of chemistry.
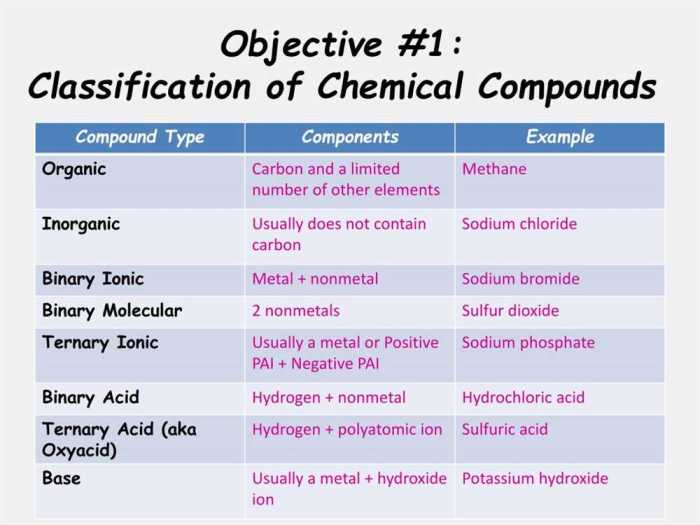
1. Compound Introduction
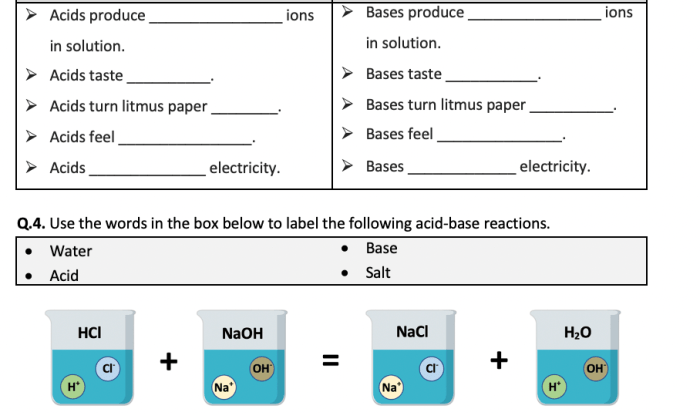
Chemical compounds are substances formed when two or more elements combine in a fixed ratio. They can be classified into various types, including ionic compounds, covalent compounds, and molecular compounds. Chemical compounds play a crucial role in our daily lives, forming the basis of many materials and products we use.
2. Sweet 16 Chemistry Compounds
- Water (H2O) : Essential for life, solvent, and reaction medium.
- Sodium chloride (NaCl): Common table salt, electrolyte, and food preservative.
- Carbon dioxide (CO2) : Greenhouse gas, used in carbonated drinks and fire extinguishers.
- Glucose (C6H 12O 6) : Primary energy source for living organisms.
- Ethanol (C2H 5OH) : Alcohol, solvent, and fuel.
- Sulfuric acid (H2SO 4) : Highly corrosive acid, used in batteries and fertilizers.
- Hydrochloric acid (HCl): Strong acid, used in metalworking and food processing.
- Ammonia (NH3) : Alkaline gas, used in fertilizers and cleaning products.
- Sodium hydroxide (NaOH): Strong base, used in soap and detergent production.
- Calcium carbonate (CaCO3) : Found in limestone, used in construction and antacids.
- Magnesium sulfate (MgSO4) : Epsom salt, used as a laxative and fertilizer.
- Potassium permanganate (KMnO4) : Oxidizing agent, used in water purification and wound disinfection.
- Sodium bicarbonate (NaHCO3) : Baking soda, used as a leavening agent and antacid.
- Potassium nitrate (KNO3) : Fertilizer, used in gunpowder and fireworks.
- Copper sulfate (CuSO4) : Blue vitriol, used as a fungicide and wood preservative.
- Iron(III) chloride (FeCl3) : Coagulant, used in water treatment and etching.
3. Tournament Structure
The tournament will consist of multiple rounds, with the top compounds advancing to each subsequent round based on their properties and applications. The selection process will consider factors such as their impact on daily life, industrial uses, and scientific significance.
The tournament structure will be presented in a visually appealing bracket system.
Frequently Asked Questions: Sweet 16 Chemistry Compound Tournament
What is the purpose of the Sweet 16 Chemistry Compound Tournament?
The tournament aims to showcase the diverse properties, applications, and interactions of sixteen key chemical compounds.
How were the sixteen compounds selected?
The compounds were chosen based on their historical significance, industrial relevance, and scientific importance.
What criteria were used to determine the winner of each round?
Compounds advanced based on their versatility, impact on society, and potential for future discoveries.